The Kalli Patent and Frequency Therapy
The Kaali patent for blood electrification would probably still be lying dormant in the archives of the Patent Office today, had it not been for a certain Dr. Robert Beck, who built on this patent, analysed and refined the findings, and based on this developed his Beck blood zapper.
The history of HIV began in 1981 when, in June, UCLA (University of California Los Angeles) scientist Michael Gottlieb reported an unusual constellation of fungal infections and pneumonia in five apparently healthy, young, homosexual men from Los Angeles in a report for the Disease Control Convention.
In 1985, the FDA approved the first HIV antibody test.
In 1989, the FDA approved the distribution of pentamidine for the prophylaxis of PCP (Pneumocystis pneumonia).
During this time, there was worldwide research into the treatment of HIV and this is where the story of the Kaali patent for blood electrification began.
Two researchers at the Albert Einstein College of Medicine in New York were experimenting with HI viruses in the Petri dish. They were Dr. Steven Kaali and his research colleague at the time, Dr. William Lyman, who, however, was no longer named when the patent was filed.
The two scientists found that HI viruses could be deactivated with an extremely low current.
The results of their months of research were announced by the two scientists to an international audience on 14 March 1991 during the First International Symposium of Combinations Therapies (First International Congress in AIDS Research).
The experimental set-up as the basis for the Kaali patent application was as follows:
A Petri dish contained HI viruses and white blood cells.
The contents of the Petri dish were exposed to a weak electric current.
In response to the current, the infectivity of the HI viruses was reduced by up to 95%.
Since blood cells are much more robust electrophysiologically than viruses, currents of 50 to 100 microamperes are sufficient to weaken the pathogens, although the blood cells themselves are not damaged.
The HI viruses are not directly destroyed by the electric current, but their outer protein coat is impaired in such a way that they can no longer produce the enzyme "reverse transcriptase (RT)".
But it is precisely this enzyme "reverse transcriptase (RT)" that HI viruses need to be able to penetrate human body cells.
This is because the RT enzyme is designed to break open the T-body cells and change the DNA of these cells in such a way that the manipulated DNA of the body cell causes the uncontrolled production of more and more new HI viruses.
Due to the blood electrification, the HI viruses were no longer able to produce the RT enzyme that is important for them. Since they no longer had this enzyme after the electrical treatment, they could no longer penetrate the host cells. They had (according to the Kaali patent) lost their destructive ability to destroy the DNA of healthy body cells and to weaken and ultimately destroy the human immune system.
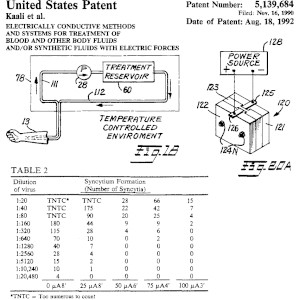
The Kaali Patent with No. 5,139,684
On 23 February 1993, the US Patent Office granted Dr Steven Kaali patent No. 5,188,738.
A link to it can be found here: Google Patent Archive.
The Kaali patent was granted on the theory of blood electrification and describes two methods for killing bacteria, viruses, fungi and parasites by introducing a specific alternating current into a virus-infected body fluid (e.g. blood).
According to US patent law, the submitter of a patent application must prove to the US Patent Office the effectiveness of the newly invented blood electrification in the listed experiment, in particular whether the described neutralisation of the HI viruses was also successful.
In order to obtain this patent from the US Patent Office, Dr. Kaali and his co-inventors had to prove that the described discovery also worked in practice, otherwise the patent would never have been granted.
FDA and AMA on the Kaali patent
The two-stage application of HIV blood electrification experimentally demonstrated by Dr. Kaali, namely the "in vitro treatment of HIV infected blood by out-of-body blood washing or/and by surgical implantation and de-implantation of mini-blood purifiers, met with high rejection from the FDA and the AMA due to the high costs and the unacceptable permanent monthly surgical interventions.
Kaali subsequently developed a device with a small battery and two tiny electrodes that could be implanted directly into an artery in the arm and leg.
However, monthly surgical procedures had to be performed to reposition the electrodes over and over again.
The costs for the American health system would have been enormous.
$5,000 per patient and month would have had to be reckoned with. Furthermore, it had to be taken into account that a decisive positive change in the patient could only be noticed after about 6 to 7 months.
The FDA (Federal Drug Administration) as the highest US-American health authority and the AMA (American Medical Administration), after an intensive review of the Kaali patent, came up with the following ethically and financially justified rejection:
- The "in vitro" method was ethically unacceptable
- The patient would have to endure unacceptable permanent interventions
- The surgical effort would be enormous
- This would result in considerable risks for the patient
- The costs for each implantation would amount to more than $ 5,000
- In view of the many HIV infections (1993), this would ruin the American health care system
Conclusion:
Based on US Patent 5,139,684, it has been researched and documented that blood electrification works on viruses, bacteria, etc. The necessary scientific evidence was attached to the patent specification.
Due to the technology of the time, the patent could not be applied to patients in this form, therefore it was not applied in a medical sense.
However, Dr. Robert Beck used this patent as the basis for his further research and some time later launched his Beck blood zapper on the market.
This device was small and handy, inexpensive and could be used on patients without restrictions.
